What is Methane (CH4)
Methane (CH4) is a colorless, odorless, and highly flammable gas that is the simplest hydrocarbon, consisting of one carbon atom bonded to four hydrogen atoms. It is a significant component of natural gas and plays a critical role in energy production, environmental concerns, and industrial processes. This article delves into the chemical nature, sources, uses, and environmental impact of methane, as well as methods for managing its emissions.
Chemical Properties of Methane
Methane’s molecular structure and properties make it a unique compound with wide applications:
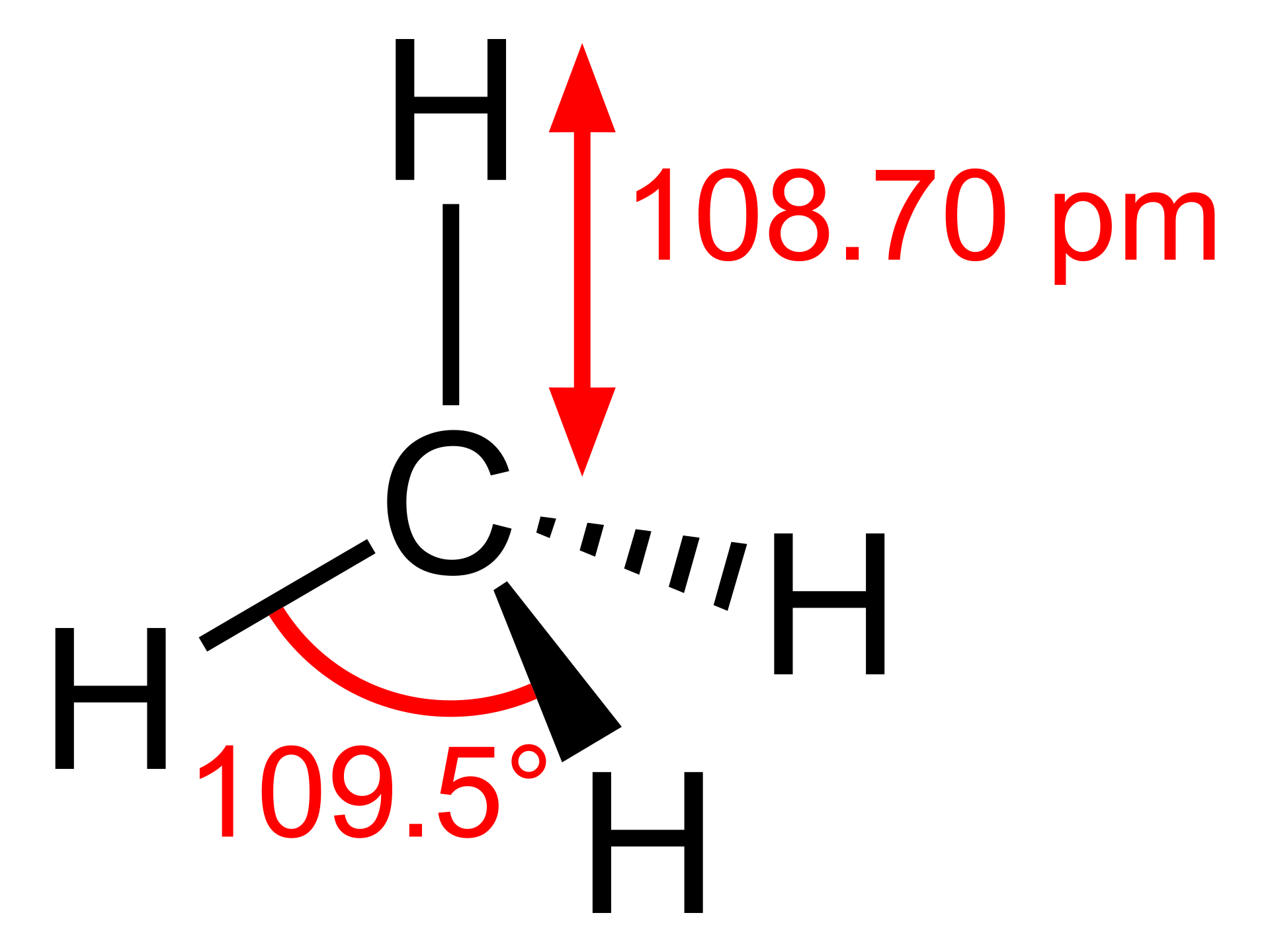 (Sources of wikipedia)
(Sources of wikipedia)- 1.Molecular Formula: CH4
- 2.Molar Mass: 16.04 g/mol
- 3.Physical State: Gas at room temperature and pressure.
- 4.Boiling Point: -161.5°C (-258.7°F)
- 5.Density: 0.656 kg/m³ (at 0°C and 1 atm)
Methane is non-toxic in small quantities but can displace oxygen in confined spaces, leading to asphyxiation. Its combustion produces carbon dioxide (CO₂) and water (H₂O), releasing significant energy.
Sources of Methane
Methane originates from both natural and human-made sources:
Natural Sources
- 1.Wetlands: The largest natural source of methane, produced by anaerobic decomposition of organic matter in waterlogged soils.
- 2.Termites: Emit methane as a by-product of digesting cellulose.
- 3.Oceans: Methane is released from microbial activity in sediments and oceanic vents.
- 4.Permafrost: As global temperatures rise, melting permafrost releases stored methane.
Anthropogenic Sources
- 1.Agriculture: Livestock, particularly cattle, produce methane during digestion (enteric fermentation). Rice paddies also emit methane through anaerobic decomposition.
- .Fossil Fuels: Extraction, processing, and transport of natural gas, oil, and coal release methane.
- 3.Landfills: Organic waste decomposing under anaerobic conditions produces methane.
- 4.Industrial Processes: Some industries, like petrochemical production, emit methane as a by-product.
Uses of Methane
Methane is a versatile fuel and chemical feedstock with applications across multiple industries:
1. Energy Production
- Natural Gas: Methane is the primary component of natural gas, which is used to generate electricity, heat homes, and power industrial facilities.
- Compressed Natural Gas (CNG): Methane is used as a clean-burning alternative fuel for vehicles.
2. Industrial Applications
- Chemical Production: Methane serves as a precursor for producing hydrogen, methanol, and other organic chemicals.
- Ammonia Synthesis: It is a key raw material in the Haber-Bosch process for ammonia production.
3. Other Uses
- Fuel Cells: Methane powers solid oxide fuel cells, producing electricity with high efficiency.
- Rocket Fuel: Methane is increasingly used as a propellant in space exploration.
Methane and the Environment
Methane is both an energy resource and a potent greenhouse gas (GHG). Its environmental implications are significant:
1. Greenhouse Gas Impact
Methane is approximately 25-28 times more effective than CO₂ at trapping heat in the atmosphere over a 100-year period. Despite its shorter atmospheric lifetime (~12 years), it contributes significantly to global warming.
2. Climate Feedback Loops
- Permafrost Thawing: Rising temperatures release methane trapped in Arctic permafrost, accelerating climate change.
- Hydrates: Methane stored in oceanic hydrate deposits could be destabilized by warming oceans, releasing massive amounts of the gas.
3. Air Quality and Public Health
- Ozone Formation: Methane contributes to the formation of ground-level ozone, a harmful pollutant.
- Explosive Risks: Methane buildup in confined spaces poses explosion hazards.
How to Know You Have Methane Poisoning?
While methane is non-toxic at low concentrations, it can pose serious risks when present in high amounts, particularly in confined spaces. Methane poisoning is primarily due to oxygen displacement, leading to asphyxiation.
Symptoms of Methane Exposure
- 1.Shortness of breath
- 2.Dizziness and confusion
- 3.Nausea or vomiting
- 4.Headache
- 5.Fatigue
- 6.Loss of consciousness in severe cases
If methane exposure is suspected, immediate evacuation to fresh air and medical attention are essential.
Methane Workplace Exposure Limits
Regulatory bodies like OSHA (Occupational Safety and Health Administration) have established guidelines to minimize workplace exposure:
- OSHA Exposure Limit: Methane is not directly toxic, but OSHA emphasizes maintaining oxygen levels above 19.5% in confined spaces.
- Explosive Limit: Methane concentrations between 5% and 15% in air are within the flammable range.
Employers are responsible for implementing safety measures, such as gas detection systems and proper ventilation, to protect workers.
To Reduce Methane Poisoning Episodes, Some Ways Are:
1.Install Methane Detectors
Gas detection systems provide early warnings of methane leaks, allowing prompt action to mitigate risks.
2.Improve Ventilation
Ensuring adequate airflow in confined spaces prevents methane accumulation and reduces asphyxiation risks.
3.Regular Maintenance
Conduct routine checks of pipelines, storage tanks, and equipment to detect and repair leaks.
4.Employee Training
Educate workers about the risks of methane exposure and emergency response procedures.
5.Capture and Utilize Methane
Methane from landfills, agriculture, and wastewater can be captured and used as renewable energy, reducing emissions and improving safety.
6.Comply with Regulations
Follow guidelines set by regulatory bodies to ensure workplace safety and minimize environmental impact.
Winsen CH4 Sensor

MPn-4C CH4 Methane Flammable Gas Sensor
- CH4, Methane, Natural gas, marsh gas
- 300~10000ppm (methane, natural gas)
- Read More

MR007 CH4 Methane C3H8 Propane Gas Sensor
- CH4 methane C3H8 propane, combustible gas, natural gas, coal gas, LPG gas
- 0~100 LEL
- Read More

MH-T4041A Low Power Consumption Infrared Gas Sensor
- Hydrocarbon flammable gases
- 0~10% Vol optional(refer to sheet 2)
- Read More
Conclusion
Methane (CH4) is an essential yet double-edged molecule—vital as a clean energy source but harmful as a potent greenhouse gas. Understanding its properties, sources, and impacts is crucial for managing its dual role in the global energy landscape and environmental sustainability. By adopting innovative technologies, enforcing regulations, and promoting awareness, we can harness methane's potential while mitigating its environmental risks.
In the broader context of combating climate change, addressing methane emissions is not just an option but an imperative for a sustainable future.










