What Is Carbon Monoxide (CO)
Carbon monoxide (CO) is a simple yet critical chemical compound that significantly impacts both natural processes and human activities. Known for its toxicity, CO is a colorless, odorless, and tasteless gas that often goes unnoticed until it poses serious health or environmental threats. However, CO's importance extends beyond its dangers, as it plays essential roles in industrial processes, medical research, and environmental science. This article explores the origins, properties, applications, and precautions associated with carbon monoxide, offering a comprehensive understanding of its role in modern society.
Chemical and Physical Properties
Composition and Structure
Carbon monoxide is composed of one carbon atom (C) and one oxygen atom (O), connected by a triple covalent bond. This strong bond gives CO its stability under standard conditions while allowing it to be reactive in certain environments.
Physical Characteristics
- Molecular Formula: CO
- Molecular Weight: 28.01 g/mol
- Melting Point: -205°C (-337°F)
- Boiling Point: -191.5°C (-312.7°F)
- Appearance: A colorless and odorless gas that is invisible to the naked eye.
Natural Occurrence of Carbon Monoxide
Natural Sources
- 1.Volcanic Eruptions: CO is released during volcanic activity due to the high-temperature decomposition of organic materials.)
- 2.Forest Fires: The combustion of wood and plant materials generates CO as a byproduct of incomplete burning.)
- 3.Microbial Processes: Certain microbes produce CO as part of their metabolic activities.)
Atmospheric Role
CO is present in trace amounts in the Earth's atmosphere, where it interacts with other gases and affects air quality. While naturally occurring levels are generally low, human activities have significantly increased atmospheric CO concentrations.
Production of Carbon Monoxide
Industrial Sources
Carbon monoxide is often generated as a byproduct of industrial processes, including:
- 1.Combustion Engines: Incomplete combustion of hydrocarbons in engines leads to CO emissions.
- 2.Steel Manufacturing: Blast furnaces produce CO during the reduction of iron ore.
- 3.Chemical Production: CO is intentionally synthesized for use in various chemical reactions.
Laboratory Preparation
In controlled settings, CO can be produced through:
The reaction of carbon dioxide (CO₂) with carbon at high temperatures:
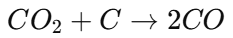
Dehydration of formic acid with sulfuric acid:
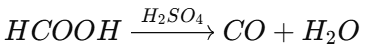
Applications of Carbon Monoxide
Despite its toxic reputation, carbon monoxide has numerous practical applications across industries:
1. Chemical Synthesis
CO is a key reactant in many industrial chemical processes:
- Methanol Production: Methanol is synthesized from CO and hydrogen, serving as a precursor for various chemicals.
- Fischer-Tropsch Synthesis: CO is used to produce synthetic fuels and hydrocarbons from hydrogen.
- Acetic Acid Manufacturing: CO is a component in carbonylation reactions to produce acetic acid.
2. Metallurgy
In the steel industry, CO acts as a reducing agent to extract iron from its ores in blast furnaces, playing a vital role in steel production.
3. Medical Research
Recent studies have revealed therapeutic applications for carbon monoxide:
- Controlled doses of CO have anti-inflammatory and anti-apoptotic effects, which may benefit organ transplantation and certain diseases.
- CO diffusion capacity tests are used to assess lung function.
4. Environmental Monitoring
- Pollution Control: CO is monitored as an indicator of air pollution from vehicular and industrial emissions.
- Climate Studies: Although CO is not a greenhouse gas, it indirectly impacts climate change by interacting with atmospheric radicals.
Health Risks and Toxicity
Carbon monoxide is infamous for its health risks due to its ability to bind with hemoglobin in the blood, forming carboxyhemoglobin. This reduces the blood's oxygen-carrying capacity, leading to hypoxia.
Symptoms of CO Poisoning
- 1.Mild Exposure: Headaches, dizziness, and nausea.
- 2.Moderate Exposure: Confusion, shortness of breath, and chest pain.
- 3.Severe Exposure: Loss of consciousness, organ damage, and death.
Sources of CO Poisoning
- Malfunctioning heaters or stoves.
- Exhaust from vehicles in enclosed spaces.
- Industrial accidents involving CO leaks.
How to Detect Carbon Monoxide
Electrochemical CO Sensor

MEu-2CO Carbon Monoxide Sensor Over 7 years lifetime
- Carbon monoxide(CO)
- 0~500ppm,Max Range 1000ppm
- Read More
Semiconductor CO Sensor

MP-9 CO/CH4 Semiconductor Flat Surfaced Gas Sensor
- CO,CH4
- 50-1000ppm(CO),300-10000ppm(CH4)
- Read More
Conclusion
Carbon monoxide is a complex compound that plays a multifaceted role in human life. While it poses significant health and environmental risks, its applications in industry, medicine, and research demonstrate its importance in advancing technology and science. By understanding the properties, applications, and safety measures associated with carbon monoxide, we can responsibly harness its potential while mitigating its dangers. Whether in chemical manufacturing, medical innovation, or environmental management, carbon monoxide remains an essential component of modern civilization.














